Abstract
Introduction
Antiphospholipid syndrome (APS) is an autoimmune condition characterized by the presence of antiphospholipid antibodies (aPL) and clinical outcomes of thrombosis and obstetric morbidity. APS is more common in patients with systemic lupus erythematous (SLE), with persistently positive aPLs being found in 20-30% of SLE patients. The revised Sapporo lab criteria for APS includes one or more of a positive lupus anticoagulant (LAC), anti-beta 2 glycoprotein 1 (aβ2GP1), or anticardiolipin (aCL). Antibodies must be persistently positive at >99 th percentile over at least 12 weeks (Miyakis et al. J Thromb Haemost, 2006). IgG anti-phosphatidylserine/prothrombin complex (aPS/PT), IgM aPS/PT, and IgG anti-domain 1β2GP1 (aD1β2GP1) are novel aPLs that have been associated with thrombosis, however, conclusive data is still lacking, and it remains unclear how best to incorporate these novel autoantibodies into clinical decision making. The aims of this study were to assess whether the non-criteria IgG aPS/PT, IgM aPS/PT, or IgG aD1 β2GP1 were associated with an increased risk of a first-time thrombotic event in SLE patients. By better understanding the risk of thrombosis in patients with novel aPLs, independent of their association with established criteria aPLs, we can better counsel patients on individual risk of thrombosis.
Methods
A retrospective chart review was performed on all participants enrolled in the SouThern Alberta Registry for Lupus EryThematosus (STARLET) database. STARLET is a prevalent cohort of 348 adult patients fulfilling American College of Rheumatology (ACR) or Systemic Lupus International Collaborating Clinics (SLICC) classification criteria for SLE. Serology results for LAC, IgG aCL, IgG aβ2GP1, IgG aPS/PT, IgM aPS/PT, and IgG aD1β2GP1 were recorded over the patient's disease course. Anti-PS/PT antibodies were measured by an enzyme-linked immunosorbent assay and IgG aD1β2GP1 by a chemiluminescence immunoassay. Electronic medical records were reviewed from March 2006 to January 2021 for first-time objectively confirmed venous, arterial, and small vessel thrombotic events, as defined by the revised Sapporo criteria. Libman-Sacks endocarditis, livedo reticularis, superficial vein thrombosis and transient ischemic attacks were not included as thrombotic events. Discrepancies in outcomes were resolved by consensus.
Results
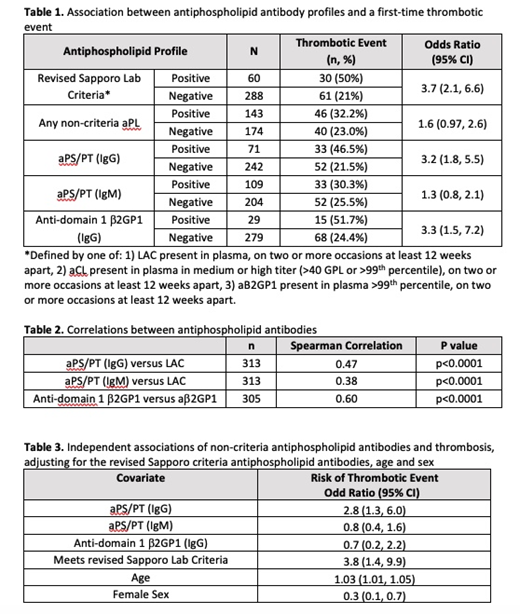
Among patients in the STARLET database, 91.8% were women, 65.5% were White, and the mean age was 42.7 (SD 15). Of 348 patients, 60 (17%) met the revised Sapporo lab criteria, and of those 30 (50%) had a thrombotic event. Among patients who met the revised Sapporo lab criteria, there was an increased risk of thrombosis (OR 3.7, 95% CI, 2.1-6.6).
Among the 143 patients that had at least one positive non-criteria autoantibody, 46 (32.2%) had a thrombotic event (OR 1.6, 95% CI 0.97-2.6) (Table 1). Univariate analysis for the association between each non-criteria antibody type and thrombosis are presented in Table 1.
There were statistically significant correlations between the revised Sapporo criteria aPLs and non-criteria aPLs; the IgG aPS/PT had a stronger correlation to LAC (Spearman=0.47) than the IgM aPS/PT (Spearman=0.38) (Table 2).
In a multivariate analysis that controlled for age, sex, and meeting the revised Sapporo lab criteria, only the non-criteria antibody IgG aPS/PT was associated with an increased risk of thrombosis with an OR of 2.8 (95% CI, 1.3-6.0) (Table 3). There were 35 patients in our cohort that did not meet revised Sapporo lab criteria that had a positive IgG aPS/PT and of these patients 8 (22.9%) had a thrombotic event.
Conclusions
In this cohort of SLE patients, IgG aPS/PT and IgG aD1 β2GP1 were associated with an increased risk of thrombosis, and both antibodies were correlated with previously established aPLs. In our multivariate analysis, only the IgG aPS/PT non-criteria antibody was associated with an increased risk of thrombosis, independent of revised Sapporo criteria aPLs, with an OR of 2.8 (95% CI, 1.3-6.0). Not all patients in the STARLET database had non-criteria aPL autoantibodies measured, and so a smaller sample size may have affected our ability to detect associations with thrombosis. Given the limited data in the area, this valuable information can help inform clinician and patient decision making and provide further guidance on the role of non-criteria antibody testing for patients with SLE.
Barber: AbbVie: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; GlaxoSmithKline: Consultancy, Honoraria; Sanofi Genzyme: Consultancy, Honoraria. Fritzler: MitogenDx Laboratory: Current Employment; Werfen International: Consultancy; Aesku Group: Consultancy; Alexion Canada: Consultancy. Skeith: CSL Behring: Research Funding; Leo Pharma: Honoraria; Sanofi: Honoraria.